Abstract
Purpose:
To assess the hematologic toxicity of PARP inhibitors (PARPi) treatment, and provide insights into the diagnosis and management of therapy-related myeloid neoplasms (t-MN) following PARPi.
Experimental Design:
In a leading cancer center, we compared the profile of 13 t-MN diagnosis among 37 ovarian cancer (OC) patients treated with PARPi referred for hematological consultation. Next, we compared these 13 t-MN post PARPi among 37 t-MN post OC. Finally, we described the specificities of 69 t-MN post PARPi treated for gynecologic cancer (breast and/or ovarian cancer) in a national observatory.
Results:
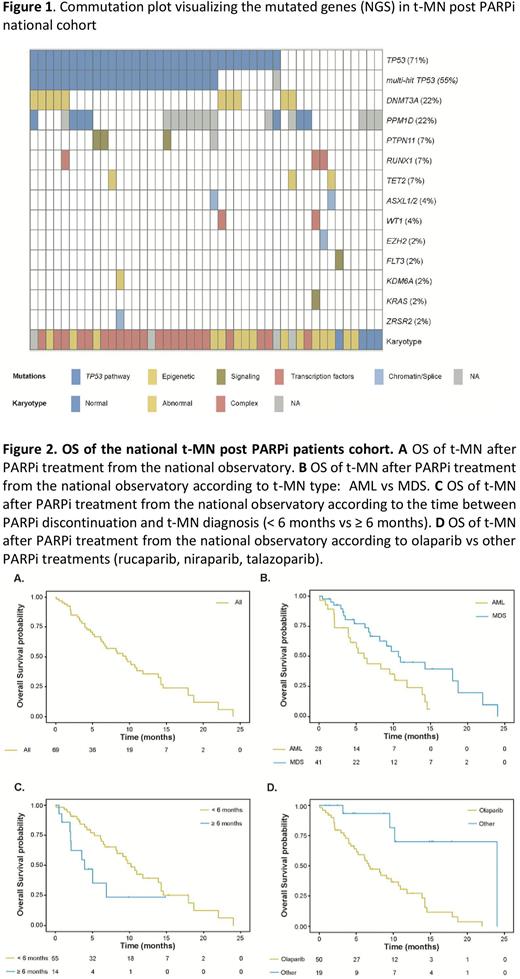
From 2016 to 2021, cumulative incidence of t-MNs was 3.5% (13/373) among OC patients treated with a PARPi. At the time of referral for hematological consultation, patients who developed t-MNs had a longer PARPi exposure (9 months vs. 3, p= 0.01), lower platelet count (74 vs. 173 G/L, p=0.0005), and more cytopenias (2 vs 1, p=0.0005). After NGS evaluation of patients without t-MN, 5 patients (13.5%) were finally classified as CHIP (clonal hematopoiesis of indeterminate potential), 7 (18.9%) as CCUS (clonal cytopenia of undetermined significance) and 2 (5%) as ICUS (idiopathic cytopenia of undetermined significance). Compared to t-MN not exposed to PARPi, t-MN-PARPi patients had more BRCA1/2 germline mutation (61.5% vs 0% p=0.03) but similar OS [8.2 (95% CI: 2.03-18.7) months in the t-MN-PARPi group compared to 6.1 (95% CI: 3-12.2) months in the t-MN-non-PARPi group; p=0.8]. In the national observatory, t-MN post PARPi had mostly a complex karyotype (61%) associated with a high rate of TP53 mutation (71%) with a median VAF of 41% (IQR, 17-67) and 75% of multi-hit mutations (Figure 1). IPSS score was "Low and Intermediate-1" and "Intermediate-2 and High" MDS groups for 50% of patients, respectively. AMLs were mainly considered as adverse (75%) according to the ELN 2017 classification. Median OS from t-MN diagnosis was 9.7 months (95% CI: 5.3-13.9). In multivariate analysis, a longer time between the end of PARPi and t-NM (HR 1.046, p=0.02), olaparib treatment compared to others PARPi (HR 5.82, p=0.003) and AML (HR 2.485, p=0.01) were associated with shorter OS independently of the BRCA1/2 status (Figure 2).
Conclusions: In a large series, we described a high incidence of t-MN post PARPi associated with unfavorable cytogenetic and molecular abnormalities leading to poor OS. Time to diagnose t-MN after PARPi interruption seems to influence OS, suggesting the importance of early diagnosis. Particular consideration should be done on BRCA1/2 patients, and in case of thrombocytopenia or more than one cytopenia and relatively long PARPi exposure.
Disclosures
Garciaz:Abbvie: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Servier: Consultancy, Honoraria. Etienne:Incyte Biosciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Gastaud:Abbvie: Consultancy; GSK: Consultancy; Celgene/BMS: Consultancy; Pfizer: Consultancy. Dumas:Janssen: Honoraria; BMS Celgene: Honoraria; Abbvie: Honoraria; Jazz Pharmaceuticals: Honoraria; Daiichi-Sankyo: Honoraria; Astellas: Honoraria. Gourin:Blueprint Medicines Corporation: Other: Fees for advisory boards; AbbVie: Other: Fees for advisory boards. Fenaux:BMS: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Recher:Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, MaatPharma, Astellas, Roche, Iqvia, Daiichi-Sankyo: Research Funding; AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, BMS-Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Roche, Takeda, Servier, Pfizer: Other: Advisory role; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dombret:Servier: Honoraria; Incyte: Honoraria. Marzac:Astellas: Honoraria. Leary:AZ: Research Funding; Clovis: Research Funding; MSD: Other: Travel to congress , Research Funding; GSK: Research Funding. Micol:Abbvie: Honoraria; AstraZeneca: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Astellas: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal